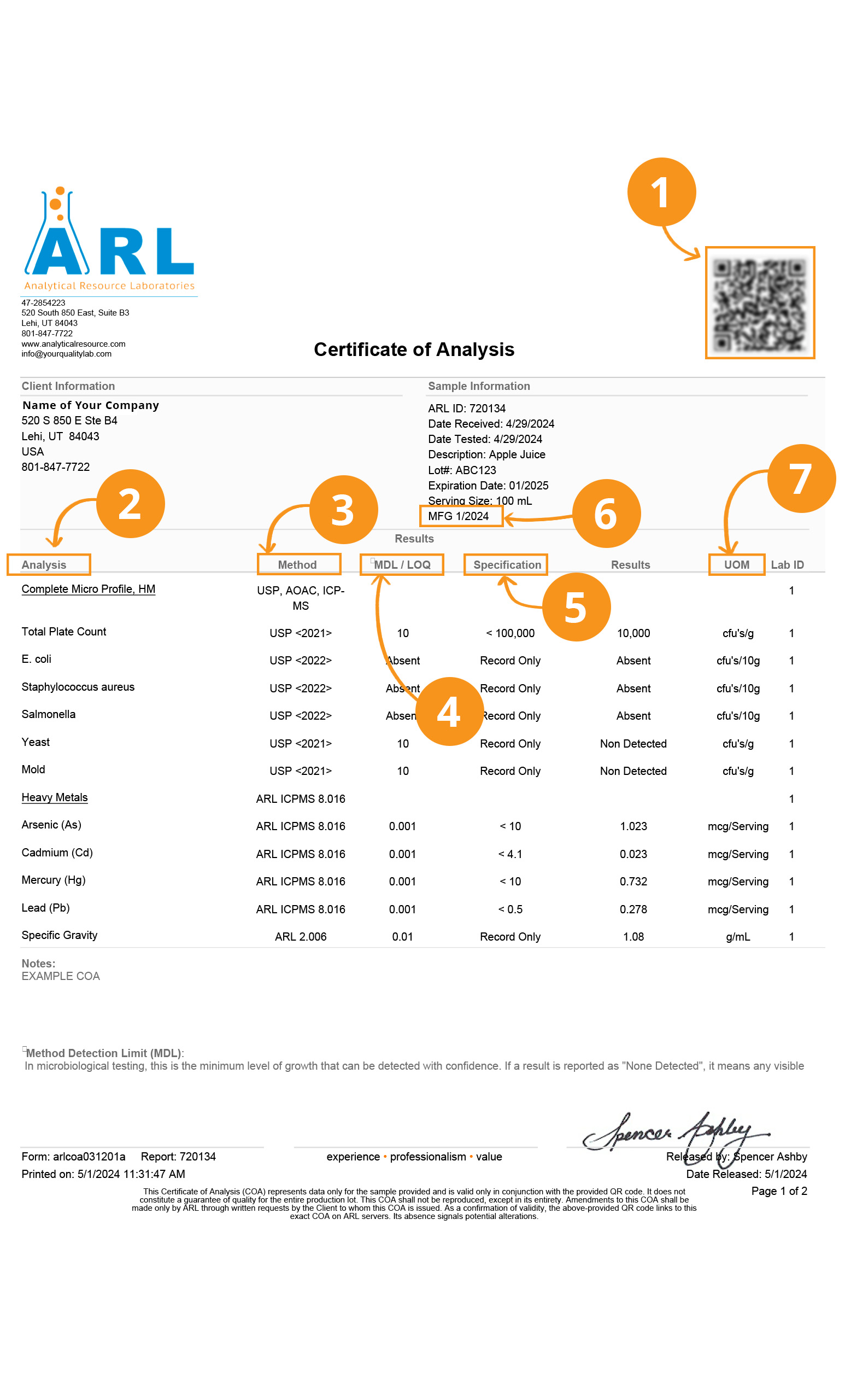
What You Need to Know About Certificates of Analysis
Certificates of Analysis (COAs) relay test data from the laboratory to the organization that submitted the sample. The information contained within a COA helps our partners make wise decisions about the suitability of their products according to their quality goals and objectives. Certificates come in many formats, such as a report narrative, a table, or graphically using bar graphs or charts. Regardless of COA format, there are some essential characteristics to look for when examining a COA from a 3rd party lab, namely accreditations and report clarity.